Adjunctive hyperbaric oxygen treatment for necrotising soft-tissue infections: A systematic review and meta-analysis
Morten Hedetoft, Michael H Bennett, Ole Hyldegaard
Appendices 1–10
Appendix 1
Database search string
MedLine:
#1 Exp hyperbaric oxygenation/or HYPERBARIC OXYGEN$.mp
#2 HBO$T.mp [mp=title, original titile, abstract, name of substance word, subject heading word]
#3 (hyperbaric adj3 oxygen$).mp [mp=title, original title, abstract, name of substance word, subject heading word]
#4 #1 or #2 or #3
#5 NECROTIZING FASCIITIS.mp. or exp fasciitis, Necrotizing/
#6 FASCIITIS.mp or exp FASCIITIS/
#7 Exp NECROSIS/
#8 #6 and #7
#9 FOURNIER$ GANGRENE.mp or exp Fournier Gangrene
#10 MELENEY$ ULCER.mp
#11 CULLEN$ ULCER.mp.
#12 HOSPITAL GANGRENE.mp.
#13 PROGRESSIVE SYNERGISTIC BACTERIAL GANGRENE.mp.
#14 SUPPURATIVE FASCIITIS.mp.
#15 H?EMOLYTIC STREPTOCOCCAL GANGRENE.mp. [mp= title, original title, abstract, name of substance word, subject heading word]
#16 ACUTE DERMAL GANGRENE.mp.
#17 SYNERGISTIC NECROTIZING CELLULITIS.mp.
#18 NECROTI?ING SOFT TISSUE INFECTION$.mp. [mp=title, original title, abstract, name of substance word, subject heading word]
#19 Exp Soft Tissue Infections/
#20 #5 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or#16 or #17 or #18 or #19
#21 #4 and #20
EMBASE:
1. (HBO$ or hyperbaric oxygen$).mp.
2. exp hyperbaric oxygen/
3. or/1-2
4. exp Fournier gangrene/ or Fournier’s gangrene.mp.
5. exp Necrotizing fasciitis/ or necrotizing fasciitis.mp.
6. exp Meleney ulcer/ or Meleney ulcer.mp.
7. (Necrotizing soft tissue infection or haemolytic streptococcal gangrene).mp.
8. ( progressive synergistic bacterial gangrene or suppurative fasciitis).mp.
9. ( acute dermal gangrene or synergistic necrotizing cellulitis).mp.
10. or/4-9
11. 3 and 10
12. Randomized Controlled Trial/
13. Clinical Trial/
14. Multicenter Study/
15. Controlled Study/
16. Crossover Procedure/
17. Double Blind Procedure/
18. Single Blind Procedure/
19. exp RANDOMIZATION/
20. Major Clinical Study/
21. PLACEBO/
22. Meta Analysis/
23. phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
24. (clin$ adj25 trial$).tw.
25. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw.
26. placebo$.tw.
27. random$.tw.
28. control$.tw.
29. (meta?analys$ or systematic review$).tw.
30. (cross?over or factorial or sham? or dummy).tw.
31. ABAB design$.tw.
32. or/12-31
33. human/
34. nonhuman/
35. 33 or 34
36. 32 not 35
37. 32 and 33
38. 36 or 37
39. 11 and 38
CINAHL:
#1 (HBO* or hyperbaric oxygen*)
#2 (Fournier gangrene or Necrotizing fasc* or Meleney* or Necrotizing soft tissue infection or haemolytic streptococcal* or progressive synergistic bacterial gangrene or suppurative fasciitis or acute dermal gangrene or synergistic necrotizing cellulitis)
#3 ((control* or trial* or random* or blind* or (clinical and trial*) or (research and design) or compar* or evaluation) not (animals not human))
#4 #1 and #2 and #3
CENTRAL:
#1 MeSH descriptor Hyperbaric Oxygen explode all trees
#2 MeSH descriptor HBOT explode all trees
#3 (hyperbaric in All Text and HBOT in All Text)
#4 (#1 or #2 or #3)
#5 MeSH descriptor Necrotising fasciitis explode all trees
#6 (gangrene in All Text or Meleney in All Text)
#7 progressive synergistic bacterial gangrene in All Text or suppurative fasciitis in All Text)
#8 (#5 or #6 or #7)
#9 #4 or #8
#10 TG=COMPARATIVE-STUDY
#11 explode EVALUATION-STUDIES/ all subheadings
#12 explode FOLLOW-UP-STUDIES/ all subheadings
Appendix 2
List of hand searched journals and conference proceedings
Journals:
Undersea and Hyperbaric Medicine,
Diving and Hyperbaric Medicine
Space and Environmental Medicine Journal
Conference proceedings:
Undersea and Hyperbaric Medical Society
South Pacific Underwater Medicine Society
European Undersea and Baromedical Society
International Congress of Hyperbaric Medicine
Appendix 3
MOOSE Checklist
Appendix 4
Risk of Bias Tools
Newcastle-Ottawa Scale (NOS)
The NOS consists of three subcategories (selection, comparability and outcome) with a possible maximum score of four in selection, two in comparability and three in outcome making nine points in total the maximum achievable. A good quality study consists of three or four points in selection, one or two points in comparability and two or three points in outcome. A fair quality study consists of two points in selection, one or two points in comparability and two or three points in outcome. A poor quality study consists of zero or one point in selection, zero points in comparability or zero or one point in outcome.
Risk Of Bias In Non-Randomized Studies – Of Interventions (ROBINS-I)
ROBINS-I consists of seven different bias domains (confounding, selection, classification of intervention, deviations, missing data, outcomes and reporting). Each domain includes a series of sub-assessments. A comprehensive guideline poses questions to facilitate the assessment of bias in each of these domains. Each domain is scored at low, moderate, serious or critical risk of bias. The total risk of bias of each study is lastly summed to either low (low in all domains), moderate (low or moderate in all domains), serious (at least one domain judged serious, none scored critical) or critical (at least one domain judged critical). In accordance with Cochrane methodology we excluded from meta-analysis all studies judged at critical risk of bias using the ROBINS-I system
Appendix 5
Demographic data of the included studies
Appendix 6
Study characteristics
Appendix 7
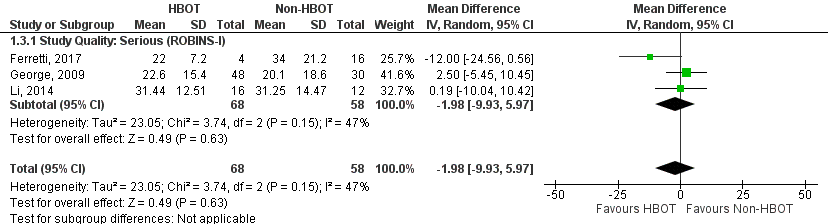
In-hospital mortality by date of study
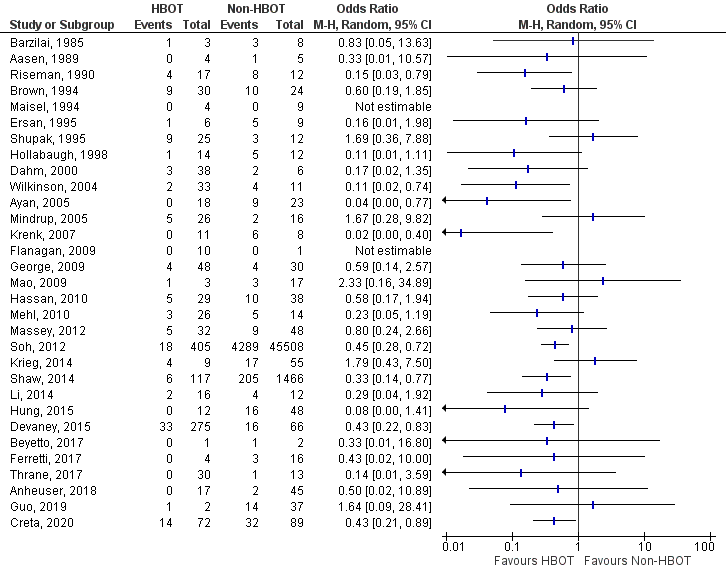
Appendix 8
Historical controls VS. Contemporary controls
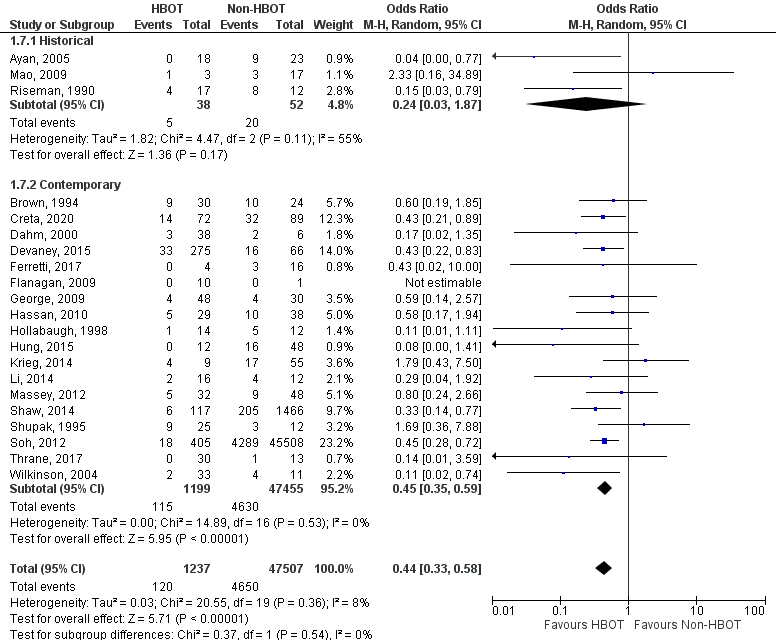
Appendix 9
Number of surgical debridements
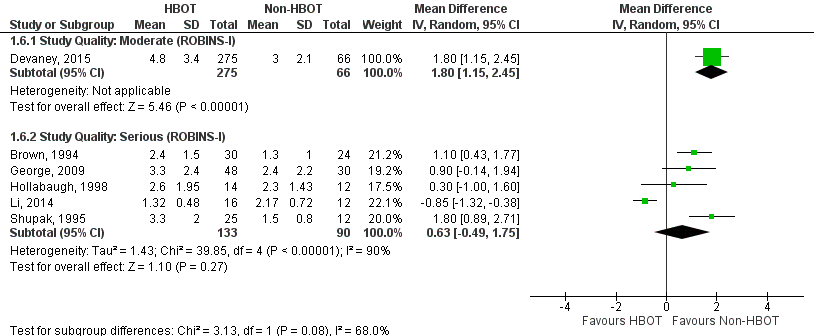
Appendix 10
Hospital length of stay